Figure 1 shows how a metabolic diversion through oxidation could give rise to Calebin A from Curcumin.
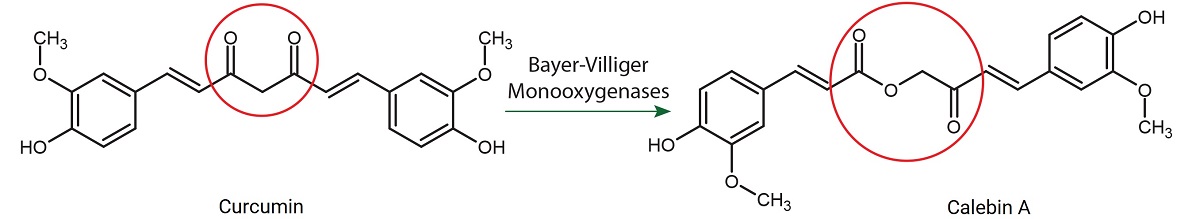
Chemistry of Calebin A: Calebin A is 3E-4-(4- Hydroxy-3-methoxyphenyl)- 2-oxo-3-buten-1-yl (2E)-3-(4-hydroxy-3-methoxyphenyl) acrylate. It is curcuminoid derived from turmeric species (Zingiberaceae family). Even though Calebin A and Curcumin originate from the same plant and co-occur in the rhizome, Calebin A has keto and ester group structural features whereas Curcumin has 1,3-diketonic structure nearly fully enolic form. Curcumin is highly conjugated with bright color but Calebin A is not. While Curcumin is unstable in higher pH environments, Calebin A is stable in alkaline conditions. Both the compounds are Michael acceptors. Both possess anti-inflammatory and antioxidant actions. Some of these differential features in the structure render Calebin A as more potent agent in the protection of PC12 cells from beta-amyloid toxicity, with higher growth inhibitory properties on human hepatoma cell line HepG2 and a potent material for alleviating risk factors associated with metabolic imbalances inclusive of underlying weight management.
